-




-
 Posture CorrectorTynor
Posture CorrectorTynor
-
 Contoured Lumbosacral BeltTynor
Contoured Lumbosacral BeltTynor
-
 Pelvic Traction Kit with
Pelvic Traction Kit with
Weight BagTynor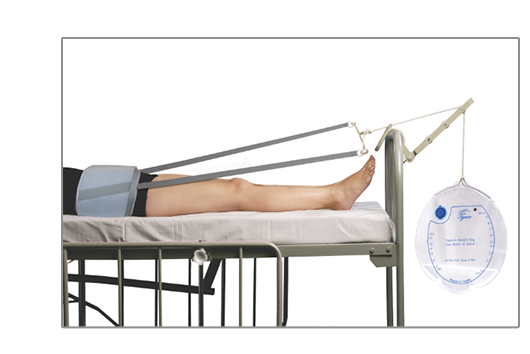
-
 Cervical Traction(Sleeping)
Cervical Traction(Sleeping)
& Weight BagTynor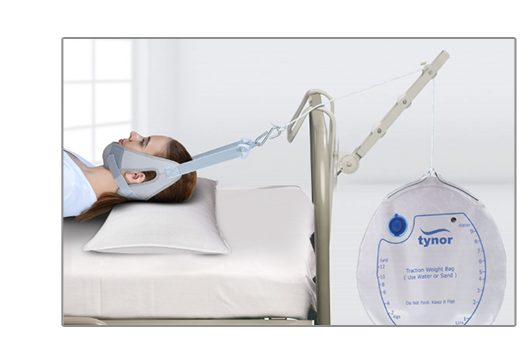
-
 Cervical Traction Kit (Sitting)
Cervical Traction Kit (Sitting)
with Weight BagTynor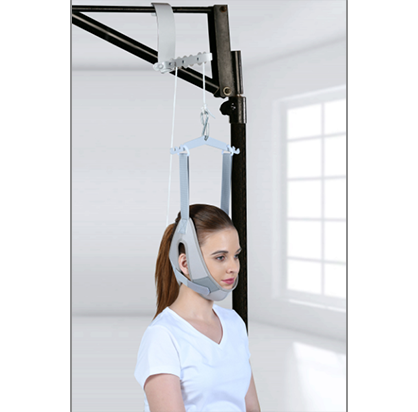
-
 Walker Invalid’s ReciprocatingTynor
Walker Invalid’s ReciprocatingTynor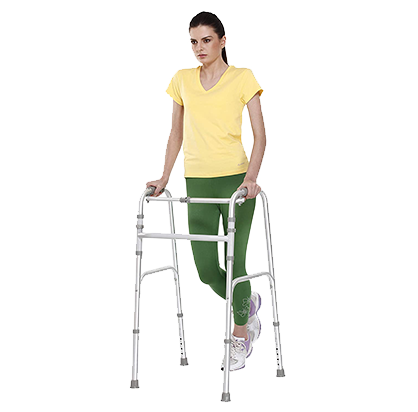
-
 Axillary Crutch (Pair)Tynor
Axillary Crutch (Pair)Tynor
-

 S.J & G.Fazul Ellahie (Pvt.) LtdPakistan Products
S.J & G.Fazul Ellahie (Pvt.) LtdPakistan Products -

 Nabiqasim Industries (Pvt) LtdPakistan Products
Nabiqasim Industries (Pvt) LtdPakistan Products -
 The IBN Sina Pharmaceutical IndustryBangladesh Products
The IBN Sina Pharmaceutical IndustryBangladesh Products
-
 Tarbros PharmaPakistan Products
Tarbros PharmaPakistan Products
-
 S.P.M CorporationVietnam Product
S.P.M CorporationVietnam Product
-
 Nan Viet BiotechnologyVietnam Product
Nan Viet BiotechnologyVietnam Product
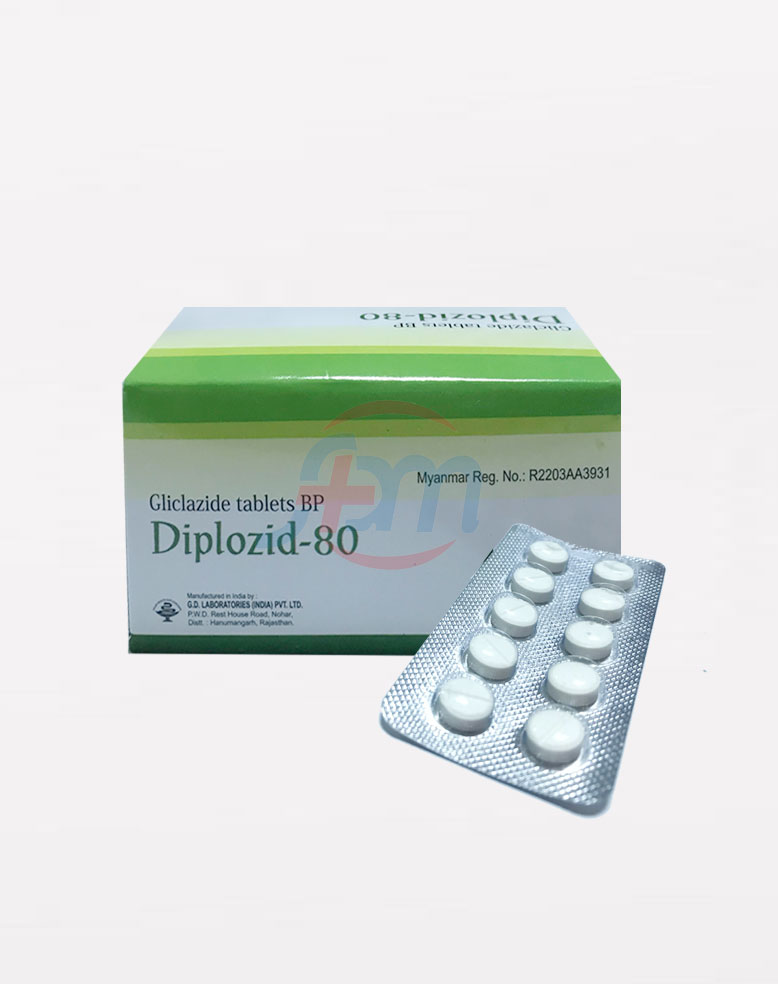
Diplozid
Diplozid-80
Composition:
Each uncoated tablet contains: Gliclazide BP 80 mg
Pharmacological Classification:
A21.2 Oral hypoglycaemics
INDICATIONS:
Treatment of non-insulin-dependent diabetes mellitus where dietary management alone has been insufficient.
WARNINGS:
The administration of oral hypoglycaemics may be associated with increased cardiovascular mortality as compared to treatment with diet alone or diet and insulin.
A reduction in dosage may be necessary in patients with renal dysfunction.
Effects on Ability to Drive and Use Machines:
Patients should be informed that their concentration may be affected if their diabetes is not satisfactorily controlled, especially at the beginning of treatment.
DOSAGE AND DIRECTIONS FOR USE:
Adults: The total daily dose may vary from 40-320 mg. The dose should be adjusted according to the individual’s response, commencing with 40-80 mg daily and increasing until adequate control is achieved. A single dose should not exceed 160 mg. When higher doses are required, gliclazide should be taken twice daily and according to the main meals of the day.In obese patients or those not showing adequate response to gliclazide alone, additional therapy may be required.
Elderly: Plasma clearance of gliclazide is not altered in the elderly and steady atate plasma levels can therefore be expected to be similar to those in adults under 65years. Clinical experience in the elderly to date shows that gliclazide is effective and well tolerated. Care should be exercised however, when prescribing sulphonylureas in the dlderly due to a possible age-related increased risk of hypoglycaemia.
Children: Gliclazide, as with other sulphonylureas, is not indicated for the treatment of juvenile onset diabetes mellitus,
Storage Condition:
Store in a dry place, at a temperature not exceeding 30ံC.
KEEP THE MEDICINE OUT OF THE REACH OF CHILDREN.
Shelf life:36 months
The preparation should not be used after expiry date.
Presentation: Blister Strip of 10 x 10 tablets.
Manufacturer's
G.D Laboratories (India) PVT.LTD
About Us
PRODUCTS
Delta Pharma
IBN Sina
Macter International
Sandar Myaing Co., Ltd
 Sandar Myaing Co.,Ltd. is one of the Myanmar's leading pharmaceutical companies, based in Yangon, with branch offices in Singapore, Mandalay and Mawlamyaing. We are well established in Myanmar and able to cover the whole country from northest Myeik Kyi Na to southest Myeik, and western Sittwe to eastern Lashio. We are authorized distributor of 15 international ... Read More
Sandar Myaing Co.,Ltd. is one of the Myanmar's leading pharmaceutical companies, based in Yangon, with branch offices in Singapore, Mandalay and Mawlamyaing. We are well established in Myanmar and able to cover the whole country from northest Myeik Kyi Na to southest Myeik, and western Sittwe to eastern Lashio. We are authorized distributor of 15 international ... Read More
Newsletter
Branch Offices
Mandalay Office
No. 354, SML Building, 81 Street, 27x28 Street, Chan Aye Tharzan Tsp, Mandalay Tsp, Myanmar
Phone : 02-4031116, 092013175
Contact us
- icon map No. 7, 108th Street, Mingalar Taung Nyunt Tsp., Yangon 11221, Myanmar.
- icon envelope Email: sandarmyaing09@gmail.com
- icon phone Phone: +95-1-291516, 297885, 09762076975